The EHVA programme is structured around 12 Work Packages:
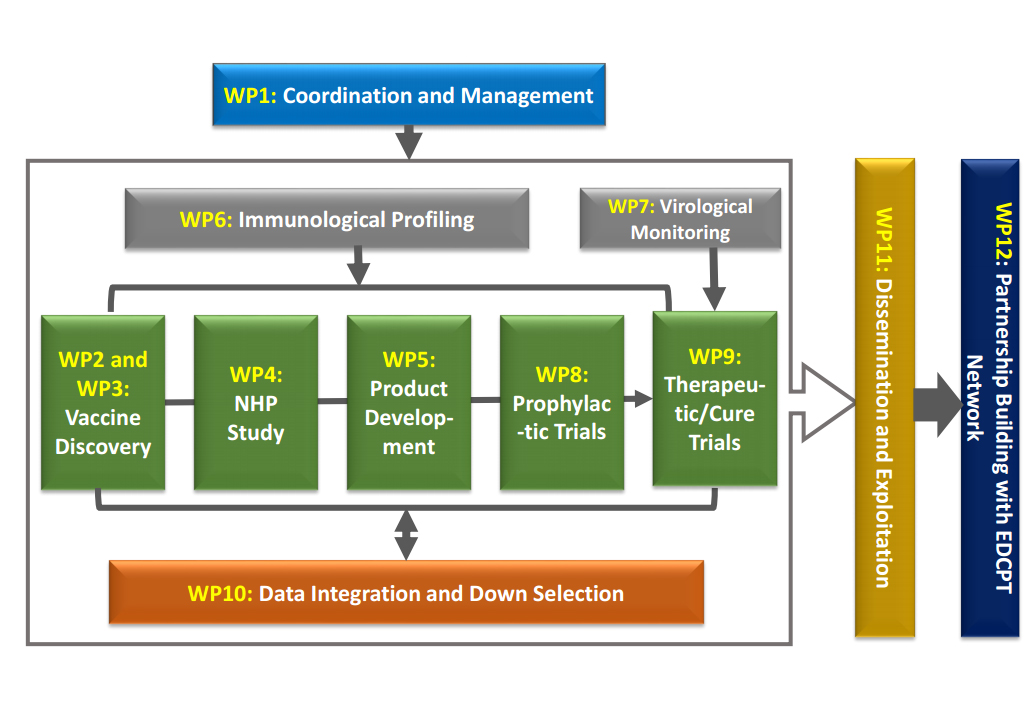
- Work Package 1 Coordination and Management
To set up an effective management framework to ensure the smooth management and coordination of the project and the achievement of planned objectives - Work Package 2 Vaccine Discovery – Novel Envelope Proteins
To improve immunogenicity of Envelop-based protein vaccines and provide insights on the role of immunological tolerance - Work Package 3 Vaccine Discovery – Non-viral and viral-vectors
To improve vaccine regimens utilizing novel non-viral and viral-vectors, evaluated in relevant models and clinical trials - Work Package 4 Non-Human Primate Studies
To optimize vaccine strategies, understand correlates of protection, and propose new approaches to accelerate the evaluation of prophylactic HIV vaccine strategies - Work Package 5 Vaccine Development
To innovative clinical trial design to facilitate rapid selection of best-in-class novel vaccine candidates and accelerate clinical development - Work Package 6 Immune Profiling
To provide assays and technologies to monitor, fully characterize and compare immune responses in different tissues induced by prophylactic and therapeutic HIV vaccination - Work Package 7 Virological Monitoring
To provide virological monitoring for therapeutic vaccine trials - Work Package 8 Prophylactic Vaccine Trials
To conduct the clinical evaluation of two novel vaccine candidates building on findings in work packages 2 and 3 - Work Package 9 Therapeutic Vaccine Trials
To develop an innovative trial platform able to rapidly evaluate (based on go/no-go criteria) vaccine candidates and novel immune-based interventions - Work Package 10 Data Integration and Down Selection
To develop an innovative approach to manage and analyse the immunological and virological data generated within the EHVA programme, and to facilitate the section of best-in-class approaches based on pre-defined immunological selection algorithms - Work Package 11 Dissemination and Exploitation
To ensure the optimal dissemination and exploitation of the results generated in the EHVA programme - Work Package 12 Partnership Building with EDCTP
To facilitate the rapid transfer of the best-in-class vaccine candidate(s) for further clinical development Sub-Saharan Africa with the support of EDCTP